Biomed Industries Unveils Promising Phase 2 Results of NA-931, First Oral Quadruple Agonist for Obesity at ENDO 2025
Biomed Industries, Inc. Unveils Promising Phase 2 Results of NA-931, First Oral Quadruple Receptor Agonist for the Treatment of Obesity at ENDO 2025
NA-931 showed excellent safety and efficacy in Phase 2 as a first-in-class oral quadruple agonist. We're excited to advance to Phase 3 to offer a more effective, well-tolerated obesity treatment.”
SAN JOSE, CA, UNITED STATES, July 17, 2025 /EINPresswire.com/ -- Biomed Industries, Inc. (Biomed) today announced that its CEO Dr. Lloyd Tran presented topline results from the Phase 2 clinical trial of NA-931, a novel oral quadruple receptor agonist for obesity, at the for ENDO 2025, July 12-15, 2025, at the Moscone Convention Center, San Francisco.— Dr. Lloyd L. Tran, CEO of Biomed
ENDO, the annual meeting of the Endocrine Society, has led advancements in hormone science and public health for more than a century. With over 7,000 global attendees, ENDO remains the premier event for endocrinology research and clinical innovation.
LANDMARK FINDINGS FOR A NOVEL ORAL THERAPY
Dr. Tran’s presentation, titled “NA-931, A Novel Quadruple IGF-1, GLP-1, GIP, and Glucagon Receptor Agonist Reduces Body Weight Without Muscle Loss”, highlighted the drug’s compelling clinical profile. NA-931 represents a breakthrough approach by simultaneously targeting four metabolic hormone pathways to achieve weight loss without compromising muscle mass.
In his remarks, Dr. Tran emphasized the central role endocrinologists play in managing obesity, noting that “imbalances in hormones such as insulin, GLP-1, and thyroid hormones can not only drive weight gain but are also exacerbated by obesity itself, creating a self-reinforcing cycle.” He underscored how NA-931’s mechanism may help break this cycle through comprehensive metabolic modulation.
PHASE 2 CLINICAL RESULTS
The Phase 2 study was a 13-week, randomized, double-blind, placebo-controlled trial evaluating the safety, tolerability, and efficacy of NA-931 in 125 adults with obesity (BMI ≥30 kg/m²) or overweight (BMI ≥27 kg/m²) with at least one weight-related comorbidity.
Body Weight Reduction
• NA-931 demonstrated dose-dependent reductions in body weight, achieving up to 13.8% mean weight loss at the 150 mg daily dose.
• This represents a 12.4% greater weight loss compared to placebo.
• 72% of NA-931-treated participants achieved ≥12% weight loss, compared to only 2% in the placebo group.
Safety and Tolerability
• Treatment-emergent adverse events (TEAEs) were generally mild and transient.
• Gastrointestinal (GI) symptoms were the most commonly reported, with 83% classified as insignificant.
• Mild nausea and vomiting were reported in 7.3%, and diarrhea in 6.3% of treated subjects.
• Importantly, no muscle loss was observed.
• There were no clinically meaningful differences in GI-related adverse events between NA-931 and placebo groups.
“The Phase 2 results of NA-931 highlight its potential as a first-in-class oral quadruple receptor agonist for weight loss, with excellent safety and efficacy,” said Dr. Lloyd L. Tran, CEO of Biomed Industries. “We are excited to advance NA-931 to Phase 3 trials and provide a more comprehensive, well-tolerated treatment for obesity.”
ADDRESSING THE GLOBAL OBESITY CRISIS
Obesity is a critical public health crisis affecting over 650 million people worldwide, with projections indicating that more than 50% of the global population could be affected by 2035. It is a key risk factor for type 2 diabetes, cardiovascular disease, non-alcoholic fatty liver disease, and chronic kidney disease.
Existing therapies often focus on single mechanisms and may lead to muscle loss or intolerable side effects. NA-931’s multi-receptor approach offers a promising new strategy by restoring metabolic balance while maintaining muscle mass and minimizing adverse events.
ABOUT NA-931
NA-931 is a first-in-class, orally active quadruple receptor agonist designed to activate IGF-1, GLP-1, GIP, and glucagon pathways. It delivers clinically significant weight loss and glycemic control, as demonstrated in both Phase 1 and Phase 2 trials—without the typical trade-offs seen in current obesity drugs. (ClinicalTrials.gov ID: NCT06564753)
ABOUT BIOMED INDUSTRIES, INC.
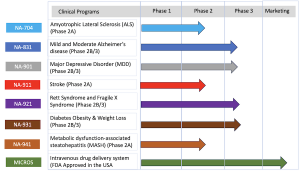
Biomed Industries, Inc. is a clinical-stage biopharmaceutical company developing next-generation therapies for unmet medical needs in metabolic, neurodegenerative, and chronic diseases. Its robust pipeline includes novel treatments for Alzheimer’s disease, ALS, Traumatic Brain Injury, Major Depressive Disorder, Diabetes, Obesity, MASH, Stroke, and rare diseases such as Rett Syndrome.
🌐 Website: www.biomedind.com
---
For media inquiries and additional information, please contact:
Michael Willis
Biomed Industries, Inc.
+1 800-824-5135
email us here
Visit us on social media:
LinkedIn
X
Biomed introduction video
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.